Synaptic Plasticity Mini-Review
 Perspective
Perspective
At the beginning of the 20th Century, Charles Sherrington coined the term synapse, which defines the specialization that allow neurons the transfer of electrical and chemical signals to other cells. Synapses are highly plastic structures, dynamically regulated to potentiate or weaken (as needed) the communication between neurons and other cell types. This particular property of synapses, referred to as synaptic plasticity, has been proposed to be the molecular substrate underlying processes of learning and memory [1]. In this mini-review, we aim to give an overview of the different forms of synaptic plasticity and their mediators and modulators. Moreover, some key compounds commonly used to study this neuronal feature will be described.
The study of chemical synapses, which consist of sophisticated presynaptic and postsynaptic terminals, has been historically predominant in the last century of investigations on synaptic plasticity. Chemical synapses will also be the topic of the present mini-review. Nevertheless, it is still important to mention the existence of electrical synapses, which connect neurons in an instantaneous fashion due to the expression of gap-junction channels and, like chemical synapses, undergo phenomena of synaptic plasticity [2].
Synaptic function
Chemical synapses are typically formed by a presynaptic specialization where the release of neurotransmitter takes place (called the active zone), and a postsynaptic area where the receptors, ready to respond to neurotransmitters, are precisely localized, (called postsynaptic density (PSD)). Depending on its morphology, synapses in the central nervous system (CNS) can be differentiated:
• Type 1 synapses (asymmetric) are normally excitatory, with numerous round shaped vesicles, a wide synaptic cleft and a prominent PSD.
• Type 2 synapses (symmetric) are normally inhibitory, with flattened vesicles, a narrower synaptic cleft and a less conspicuous PSD.
Neurotransmitter release
In the active zone of the presynaptic terminal, neurotransmitters are packed in vesicles localized in proximity to the plasma membrane. In each active zone, 100-200 synaptic vesicles of about 40 nm of diameter are clustered, each of them filled with several thousand molecules of neurotransmitters.
In the last 30 years, our understanding of the complex process underlying the release of neurotransmitters has grown considerably. It is reasonable to say that the main driving agent in the process of neurotransmitter release is calcium, as shown for the first time by Bernard Katz and Ricardo Miledi in the 1960s [3]. The depolarization induced by the arrival of an action potential, induces the opening of voltage-gated calcium channels (VGCCs), mainly N- and P/Q-type. In this moment, calcium ions enter the presynaptic terminal to exert a plethora of effects by acting as a second messenger that ultimately triggers the release of neurotransmitter molecules [4].
Postsynaptic receptors
Presynaptic, postsynaptic, perisynaptic and extrasynaptic receptors transduce the action of the neurotransmitters. Eventually, one or more of the released neurotransmitter molecules will bind to a receptor which can be either metabotropic and trigger the activation of secondary intracellular pathways, or ionotropic, thus provoking the opening of the receptor (in fact a ligand-gated ion channel) in a probabilistic manner.
When a single synapse is activated by an action potential, thus triggering the release of a neurotransmitter, a small response occurs at the level of the postsynaptic terminal. This is called an excitatory or inhibitory postsynaptic potential (EPSP or IPSP) depending on what type of current is flowing through the receptors that are activated. Although a single EPSP is normally not enough to cause the generation of an action potential in the postsynaptic neuron, the summation of many EPSPs occurring at different synaptic inputs in the same neuron could eventually depolarize the neuron to levels enough for the generation of an action potential. On the other hand, IPSPs can counteract this depolarization preventing the neuron from firing.
Many neurotransmitters (dopamine, acetylcholine, serotonin, GABA, etc.) can give rise to processes of synaptic plasticity, however it is glutamatergic synaptic plasticity that has been most extensively studied and will be reviewed here.
Glutamate acting on ionotropic receptors mediates most of excitatory transmission in the hippocampus (and rest of the brain). There are three ionotropic receptors activated by glutamate, and which constitute the essential transducers of the glutamate signal for the generation of EPSPs:
- AMPA receptors (AMPAR) are ligand-gated ion channels responsible for fast excitatory transmission in the hippocampus. In principal cells under resting conditions (basal synaptic transmission), AMPARs are the main mediators of the generation of EPSPs, allowing influx of sodium and potassium ions through the ionophore. They are tetramers composed, in physiological conditions, by a different combination (heterotetramers) of four subunits (GluA1-GluA4), all of them expressed in the hippocampus [5]. AMPARs mediate basal synaptic transmission and can be specifically inhibited using, for example, NBQX.
- NMDA receptors (NMDARs) are obligated heterodimers of GluN1 (two in native tetrameric NMDARs) and GluN2 subunits (recently a GluN3 subunit has been described). The GluN1 subunit is the crucial subunit of all NMDARs and it does not bind glutamate, but instead glycine, which functions as an essential co-agonist for the NMDAR to function; the GluN2 subunits bind glutamate [6]. Two major characteristics differentiate NMDARs from other glutamatergic receptors: their high permeability to calcium ions and their voltage-dependent blockade by extracellular magnesium. These properties determine NMDARs as key elements during the induction of synaptic plasticity.
- Kainate receptors are heterodimers composed by different combinations of five subunits (GluK1-GluK5). Some of their most interesting characteristics are their slow kinetics and their dual function acting as ionotropic receptors and triggering G protein-mediated actions. Kainate receptors can be inhibited, together with AMPARs, using CNQX.
Glutamate also activates G protein-coupled metabotropic receptors (mGluRs) which can be located both pre- and postsynaptically and transduce the glutamatergic signal to modulate neuronal excitability and synaptic transmission.
Short-term synaptic plasticity
The presynaptic terminal does not work as a passive element by simply eliciting the release of neurotransmitter as a response to an action potential. Indeed, it functions as a sort of a filter, whose properties depend on the nature of the series of action potentials incoming the terminal. These use-dependent changes in the efficacy of a presynaptic terminal to liberate neurotransmitter define a synapse specific property, known as short-term plasticity. There are different types of short-term plasticity (Table 1).
 Paired-pulse facilitation
Paired-pulse facilitation
When a pair of action potentials invades the presynaptic terminal within a short time (normally less than 1 second), depending on the specific properties of the synapse, the second action potential may evoke an EPSP which is larger than the one caused by the first action potential, a phenomenon referred to as paired-pulse facilitation (PPF) [7]. PPF is a synapse specific feature whose properties will differ among synapses depending on different factors [8] - the probability of neurotransmitter release and the calcium buffering properties in the presynaptic terminal being the most determinant. This makes PPF a very suitable model to infer whether a particular experimental manipulation is exerting its effects by acting at the level of the presynaptic terminal. In general, compounds that decrease the probability of neurotransmitter release will enhance PPF (e.g. adenosine analogs).
Diagram 1. Paired-pulse facilitation of two different synapses in the hippocampus. PPF is a synapse-specific property that strongly depends on the basal probability of neurotransmitter release. MF = Mossy fibers, AC = associational / commissural fibers (modified from [9]).
Paired-pulse depression
Contrary to PPF, synaptic depression is a use-dependent decrease in synaptic strength during two consecutive presynaptic inputs. One hypothesis is that during periods of high-frequency stimulation (HFS) there is a gradual depletion of the pool of vesicles ready to be released, thus depressing the synapse [10].
Post-tetanic potentiation
Post-tetanic potentiation (PTP) is the transient increase in synaptic strength (lasting tens of seconds to minutes) following a period of sustained HFS.
Augmentation
Augmentation is a form of plasticity similar to PTP, but of shorter duration and induced by lower frequency stimulation. The distinction between PTP and augmentation is not well defined, but it can be based on their different decay kinetics.
| Form of short-term plasticity | Decay (aprox. τ values) |
| Paired-pulse facilitation (PPF) | 50 - 300 ms |
| Augmentation | 7s |
| Post-tetanic potentiation (PTP) | Tens of seconds to minutes |
Table 1. Decay associated with different forms of short-term plasticity.
Although there is general agreement about the presynaptic nature of these phenomena, the specific mechanisms underlying PPF and other forms of short-term plasticity are still far from being definitively resolved [10].
Long-term synaptic plasticity
A complex cluster of action potentials arrives to the presynaptic terminal and a coded release of neurotransmitter takes place to induce the depolarization of the postsynaptic terminal in the form of an EPSP. Although a presynaptic component appears to partly account for some sorts of long-term plasticity, it is believed that long-term plasticity (which can last from hours until days, weeks, months or even years) is mainly mediated by processes taking place at the postsynaptic terminal.
Metabotropic receptors directly mediate the transduction of a neurotransmitter signal into intracellular changes that will affect the physiology of the neuron. However, it is fast neurotransmission, through the action of ionotropic receptors, that plays a central role in setting the stage for the events leading to long-term plasticity, and more specifically, intracellular changes in calcium concentration that constitute the molecular core of hippocampal synaptic plasticity and learning [11].
Long-term potentiation
In 1973, Tim Bliss and Terje Lømo published an article in the Journal of Physiology describing long lasting strengthened potentiation in granule cells of the dentate gyrus after repetitive brief tetanic stimulation of the perforant pathway, studied in vivo in the anaesthetized rabbit. For the first time, an experimental description of a Hebb-like synapse in the mammalian brain was given [12]. Since then, long-term potentiation (LTP) became one of the most studied models in neuroscience, yet its detailed mechanisms remain to be fully understood. The popularity of LTP is based on the proposal that it is (as well as its counterpart long-term depression) the probable molecular substrate of many forms of learning and memory [1, 13].
After a period of baseline recordings at low frequency (typically one response is elicited every 30 seconds, i.e., 0.033 Hz), which is necessary to confirm the stability of the EPSP responses, LTP can be readily induced following the application of different protocols of HFS, both in vivo and in vitro using brain slices. Common protocols include one or more trains of pulses at 100 Hz for 1 second per train or patterns consisting of shorter trains given at a theta frequency (5 Hz). NDMARs, which are normally blocked by magnesium under basal conditions, are activated during periods of HFS, giving rise to an increase of calcium in the postsynaptic terminal. After tetanisation, the EPSP is again recorded at low frequency as during baseline, and the different phases of LTP can be studied.
Depending on the induction protocol used, different forms of LTP can be triggered and normally a differentiation between NMDAR-dependent and NMDAR-independent forms of LTP is used [14].
The most studied form of LTP, NMDAR-dependent LTP, is comprised of different temporal phases whose expression also depends on the induction protocol applied [15]:
- PTP can be seen in most LTP experiments after HFS. In agreement with the presynaptic nature of this form of short-term plasticity, it can be isolated from the other components of LTP by the application of an NMDAR antagonist (e.g. D-AP5).
- Short-term potentiation (STP, also referred to as LTPa) normally decays over a period of tens of minutes. The magnitude of STP depends on the frequency used to induce plasticity: the higher the frequency the greater the magnitude. The duration of STP depends on how many stimuli were given to induce plasticity and the frequency rate used to assess basal transmission after induction: the lower the frequency the slower its decay. This has the interesting implication that when stimulation is paused just after tetanus (meaning that no stimuli are given), STP does not decay until the stimulation recommences, in other words, STP can be stored [16]. Furthermore, STP requires the activation of specific NMDARs, as it can be partially inhibited after the blockade of NMDAR-containing GluN2A (with NVP-AAM 077), GluN2B (with Ro 25-6981) or GluN2D subunits (with UBP145).
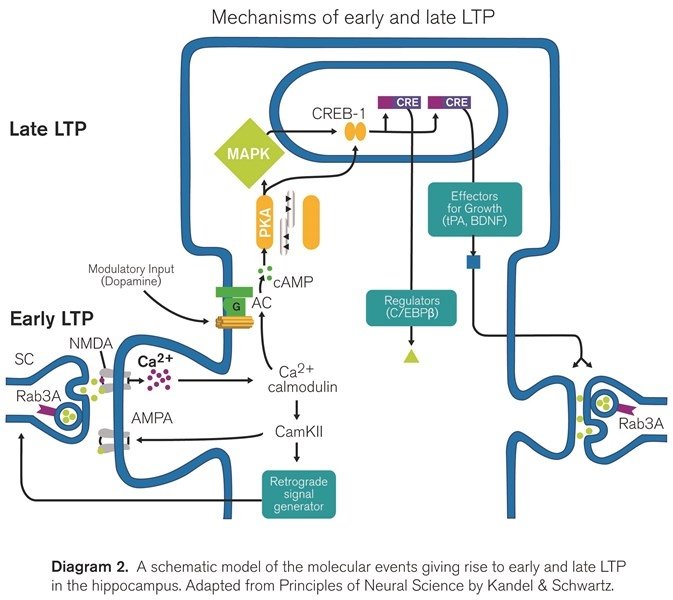
- Early LTP (also referred to as LTPb) is a component of LTP that does not require protein synthesis, but it is dependent on the activation of certain kinase proteins. The entrance of calcium through NMDARs during induction will trigger the persistent activity of different kinases such as protein kinase C, and most importantly, the calcium/calmodulin-dependent protein kinase II (CaMKII). The activated CaMKII will phosphorylate existing AMPA receptors and in this way lead to the insertion of new receptors thus potentiating the synapse.
- Late LTP (referred to as LTPc) is defined as the temporal component of LTP that is abolished by protein synthesis inhibitors (such as anisomycin) or by transcriptional blockers, responsible for the persistence of LTP during hours, days or even longer. Translation of pre-existing mRNA in the dendrites of potentiated synapses accounts for the initial stages of late LTP while transcription is necessary for generating the persistent phase of late LTP, which might include some structural changes [17]. Importantly, late LTP depends on the activity of protein kinase A [15, 18], as it can be inhibited in the presence of PKA inhibitors (e.g. KT5720). Moreover, late LTP induction also depends on the opening of calcium channels, as it can be partially inhibited after the blockade of L-type VGCCs (using for example isradipine) [19].
The pharmacological and temporal basis (in terms of duration) leading to the differentiation of these forms of LTP suggests that they are indeed different and segregated forms of LTP, however this issue is currently under intense debate [15, 20-22].
| LTP component | Duration | NMDAR | Protein kinase activity | Protein synthesis |
| PTP | 1-3 minutes | Independent | Independent | Independent |
| STP | 20-60 minutes | Dependent | Independent | Independent |
| Early LTP | 1-3 hours | Dependent | Dependent (CaMKII) | Independent |
| Late LTP | >3 hours | Dependent | Dependent (PKA) | Dependent |
Table 2. Properties of the different phases of long-term potentiation.
Long-term depression
Long-term depression (LTD) is a long-lasting decrease of synaptic strength below baseline, often classified into two main types, namely, NMDAR-dependent LTD and metabotropic glutamate receptor-dependent LTD [14]. The term depotentiation applies when LTD is induced over LTP, thus reducing it.
- In the CA1 area of the hippocampus, NMDAR-dependent LTD can be induced by the application of prolonged protocols of low frequency stimulation, e.g. 900 stimulations at 1 Hz (although other protocols can be used). It is also possible to induce this form of LTD in a chemical way by a brief application of NMDA [23].
- In the hippocampus, besides the activation of NMDAR and NMDAR-dependent LTD, glutamate can also bind to metabotropic glutamate receptors and induce mGluR-LTD [24]. The activation of the three groups of mGluRs by specific agonists and allosteric modulators (e.g. DHPG for group I mGluRs; BINA for group II; L-AP4 for group III) is enough to generate this chemical form of LTD [25].
Other forms of synaptic plasticity
Homeostatic plasticity
By driving synaptic strength to increased and decreased levels, LTP and LTD inevitably destabilize a neuron´s excitability. Homeostatic plasticity refers to the ability of neurons to adapt their intrinsic levels of excitability in response to the activity of the neuronal network containing them [26]. This is a less studied form of synaptic plasticity yet essential to maintain the functional equilibrium of neuronal networks.
Metaplasticity
Metaplasticity is often referred to as the plasticity of synaptic plasticity. More specifically, when a synapse undergoes LTP or LTD, its ability to respond to new events leading to synaptic plasticity is changed [27]. This form of plasticity is therefore dependent on the history of a neuron and is critical towards setting the stage for the establishment of new processes of LTP and LTD.
In summary, the term synaptic plasticity comprises a complex compendium of phenomena that interact in an integrated and meticulous fashion. Altogether, they constitute the cellular basis of learning and memory ultimately leading to the formation of neural engrams [28]. Although our knowledge of synaptic plasticity has been hugely increased in the last century, the complexities of memory formation remain a complete mystery. The challenge is there, ready for a new generation of brain research to happen.
References
1. Takeuchi T, Duszkiewicz AJ, Morris RG. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130288. doi: 10.1098/rstb.2013.0288. PMID: 24298167.
2. Curti S, O'Brien J. Characteristics and plasticity of electrical synaptic transmission. BMC Cell Biol. 2016;17 Suppl 1:13. doi: 10.1186/s12860-016-0091-y. PMID: 27230893.
3. Katz B, Miledi R. The effect of calcium on acetylcholine release from motor nerve terminals. Proc R Soc Lond B Biol Sci. 1965;161:496-503. PMID: 14278410.
4. Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80(3):675-90. doi: 10.1016/j.neuron.2013.10.022. PMID: 24183019.
5. Greger IH, Watson JF, Cull-Candy SG. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron. 2017;94(4):713-30. doi: 10.1016/j.neuron.2017.04.009. PMID: 28521126.
6. Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327-35. PMID: 11399431.
7. Regehr WG. Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol. 2012;4(7):a005702. doi: 10.1101/cshperspect.a005702. PMID: 22751149.
8. Blitz DM, Foster KA, Regehr WG. Short-term synaptic plasticity: a comparison of two synapses. Nat Rev Neurosci. 2004;5(8):630-40. doi: 10.1038/nrn1475. PMID: 15263893.
9. Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93(23):13304-9. PMID: 8917586.
10. Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355-405. doi: 10.1146/annurev.physiol.64.092501.114547. PMID: 11826273.
11. Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5-21. doi: 10.1016/j.neuron.2004.09.012. PMID: 15450156.
12. Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331-56. PMID: 4727084.
13. Morris RG. NMDA receptors and memory encoding. Neuropharmacology. 2013;74:32-40. doi: 10.1016/j.neuropharm.2013.04.014. PMID: 23628345.
14. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18-41. doi: 10.1038/sj.npp.1301559. PMID: 17728696.
15. Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, et al. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130131. doi: 10.1098/rstb.2013.0131. PMID: 24298134.
16. Volianskis A, Jensen MS. Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus. J Physiol. 2003;550(Pt 2):459-92. doi: 10.1113/jphysiol.2003.044214. PMID: 12794181.
17. Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116(3):467-79. PMID: 15016380.
18. Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260(5114):1661-4. PMID: 8389057.
19. Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16(5):973-82. PMID: 8630255.
20. Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015;1621:5-16. doi: 10.1016/j.brainres.2015.01.016. PMID: 25619552.
21. Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the "long" in long-term potentiation. Trends Neurosci. 2007;30(4):167-75. doi: 10.1016/j.tins.2007.01.007. PMID: 17292975.
22. Abbas AK, Villers A, Ris L. Temporal phases of long-term potentiation (LTP): myth or fact? Rev Neurosci. 2015;26(5):507-46. doi: 10.1515/revneuro-2014-0072. PMID: 25992512.
23. Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459-73. doi: 10.1038/nrn2867. PMID: 20559335.
24. Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61(4):395-412. doi: 10.1124/pr.109.001735. PMID: 19926678.
25. Lodge D, Tidball P, Mercier MS, Lucas SJ, Hanna L, Ceolin L, et al. Antagonists reversibly reverse chemical LTD induced by group I, group II and group III metabotropic glutamate receptors. Neuropharmacology. 2013;74:135-46. doi: 10.1016/j.neuropharm.2013.03.011. PMID: 23542080.
26. Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. PMID: 22086977.
27. Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008 May;9(5):387. doi: 10.1038/nrn2356.PMID: 18401345
28. Josselyn SA, Kohler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16(9):521-34. doi: 10.1038/nrn4000. PMID: 26289572.





 Perspective
Perspective Paired-pulse facilitation
Paired-pulse facilitation