How to Choose an Acrylamide Gel Concentration for Western Blot
Introduction
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) is an essential molecular biology technique where complex mixtures of protein are separated in an acrylamide gel according to their molecular weight. Dependent on the molecular weight of the protein(s) of interest different formulations of acrylamide gel are required to gain optimal separation of proteins. Gels can either use a single concentration of acrylamide where they are optimised for a narrow range of molecular weights (see section 1) or be gradients of acrylamide concentration where they are optimised for a much wider range of weights (see section 2). Regardless of resolving gel type all experiments require a small layer of stacking gel (see section 3) which allows proteins to stack up and enter the resolving gel at the same time. This does not participate in the separation of proteins and is a constant recipe.
1. Single concentration gels
In SDS-PAGE all proteins are denatured and have a uniform negative charge applied to them therefore migration is due to difference in size between proteins. Polyacrylamide gels are a matrix of cross-linked acrylamide monomers with the tightness of the mesh dependent upon the amount of acrylamide and cross-linker present. Different sized proteins therefore require different formulations of acrylamide gel to achieve optimum separation (table 1).
|
Protein size (kDa) |
Gel Percentage (%) |
|
4-40 |
20 |
|
12-45 |
15 |
|
10-70 |
12 |
|
15-100 |
10 |
|
25-200 |
7.5 |
|
>200 |
5 |
Table 1 Suggested concentrations of acrylamide gel depending upon protein of interest size.
Where multiple proteins of differing sizes need to be separated consider using a gradient gel.
Where there is only one protein of interest or the proteins to be separated are of a similar size then a single concentration gel can be used. Generally the larger the protein the larger pore size is needed in the polyacrylamide gel and the smaller the protein the smaller the pore size (table 1). Once the gel concentration needed has been identified these can either be purchased as pre-cast gels or made in the laboratory using the recipe in table 2.
|
Reagent |
Order |
Gel concentration (%) |
|||||
|
20 |
15 |
12 |
10 |
7.5 |
5 |
||
|
dH2O |
1 |
0.93ml |
2.34ml |
3.28ml |
3.98ml |
4.78ml |
5.61ml |
|
1.5M Tris-HCl pH 8.8 |
2 |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
|
10% SDS |
3 |
100µl |
100µl |
100µl |
100µl |
100µl |
100µl |
|
30% Acrylamide/Bis (29.2:0.8) |
4 |
6.7ml |
5ml |
4ml |
3.3ml |
2.5ml |
1.67ml |
|
10% APS |
5 |
50 µl |
50 µl |
50 µl |
50 µl |
50 µl |
50 µl |
|
TEMED |
6 |
5µl |
5µl |
5µl |
5µl |
5µl |
5µl |
Table 2. Recipe for the construction of polyacrylamide resolving gels. Makes a 10ml gel. Be sure to add reagents in the correct order with APS and TEMED being added last.
CAUTION: Acrylamide is a potent neurotoxin therefore gloves should be worn at all times.
2. Variable concentration gels
If multiple proteins of significantly differing sizes need to be separated then gradient gels can be instead used. These vary the concentration of the gel along the migration path to provide optimal separation. These can be made in the laboratory but are more easily purchased.
3. Stacking gels
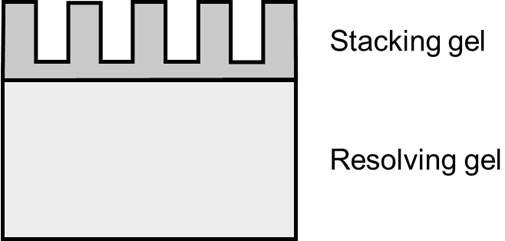
In order to line up proteins before they enter the resolving gel it is important for proteins to pass through a short layer of stacking gel (Figure 1). This allows proteins to enter the resolving gel at the same point and is the same acrylamide concentration regardless of protein size (Table 3).Figure 1. Structure of an acrylamide gel for SDS-PAGE
|
Reagent |
Order |
Volume |
|
dH2O |
1 |
3.05ml |
|
0.5M Tris-HCl pH 6.8 |
2 |
1.25ml |
|
10% SDS |
3 |
50µl |
|
30% Acrylamide/Bis (29.2:0.8) |
4 |
650µl |
|
10% APS |
5 |
25µl |
|
TEMED |
6 |
10µl |
Table 3 Recipe for acrylamide stacking gel. Makes 5ml suitable for a 10ml resolving gel. Be sure to add reagents in the correct order with APS and TEMED being added last.
CAUTION: Acrylamide is a potent neurotoxin therefore gloves should be worn at all times when making or handling the gel.
4. Protocol for pouring a constant concentration gel
1. Clean casting stand and gel holders with distilled water. Clean plates with distilled water, 90-100% ethanol and finally acetone.
2. Clean gel face of plates with pure silicone polish.
3. Construct gel mould on holder
4. Mix resolving gel (adding ammonium persulphate and TEMED last) and pour immediately (for 1mm thickness use 5ml). The resolving gel should be far enough below the top of the gel plates to allow insertion of the comb + 1cm.
5. Overlay resolving gel with water saturated butan-1-ol or water to maintain a flat surface on the resolving gel during polymerisation. Leave to set for 15-60 minutes.
Tip: Make a small amount of extra resolving gel then the progress of polymerisation can be monitored easily.
Tip: The time taken for polymerise can vary quite significantly dependent on multiple factors. If gels are taking a long time to polymerise (>60 mins) consider making or purchasing fresh ammonium persulphate and TEMED.
7. Mix stacking gel except APS and TEMED. Pour off overlay and use a tissue wick to remove the last remnants. Add APS and TEMED to stacking gel and pour (0.6ml for 7mm thickness).
8. Add the comb with no air bubbles and leave to set for approximately 30mins.
Tip: Make extra stacking gel then the progress of polymerisation can be monitored easily